Video review of the model
Video review of the model
 Our service and customer service
Our service and customer service
The release of drugs from hydrophobic bases is slower.
When making suppositories, it should be remembered that the drug substances introduced into the composition of these dosage forms in the form of aqueous solutions, absorbed much easier and faster have a local effect than the drug substances introduced in dry form. In terms of microbiological purity of preparations for rectal administration are classified by the State Pharmacopoeia to category IIIA, ie in 1 g or 1 ml of the drug should contain no more than 1000 aerobic bacteria and 100 fungi in the absence of Escherichia coli.
 Pharmaceutical Glossary
Pharmaceutical Glossary
 Technical specifications
Technical specifications
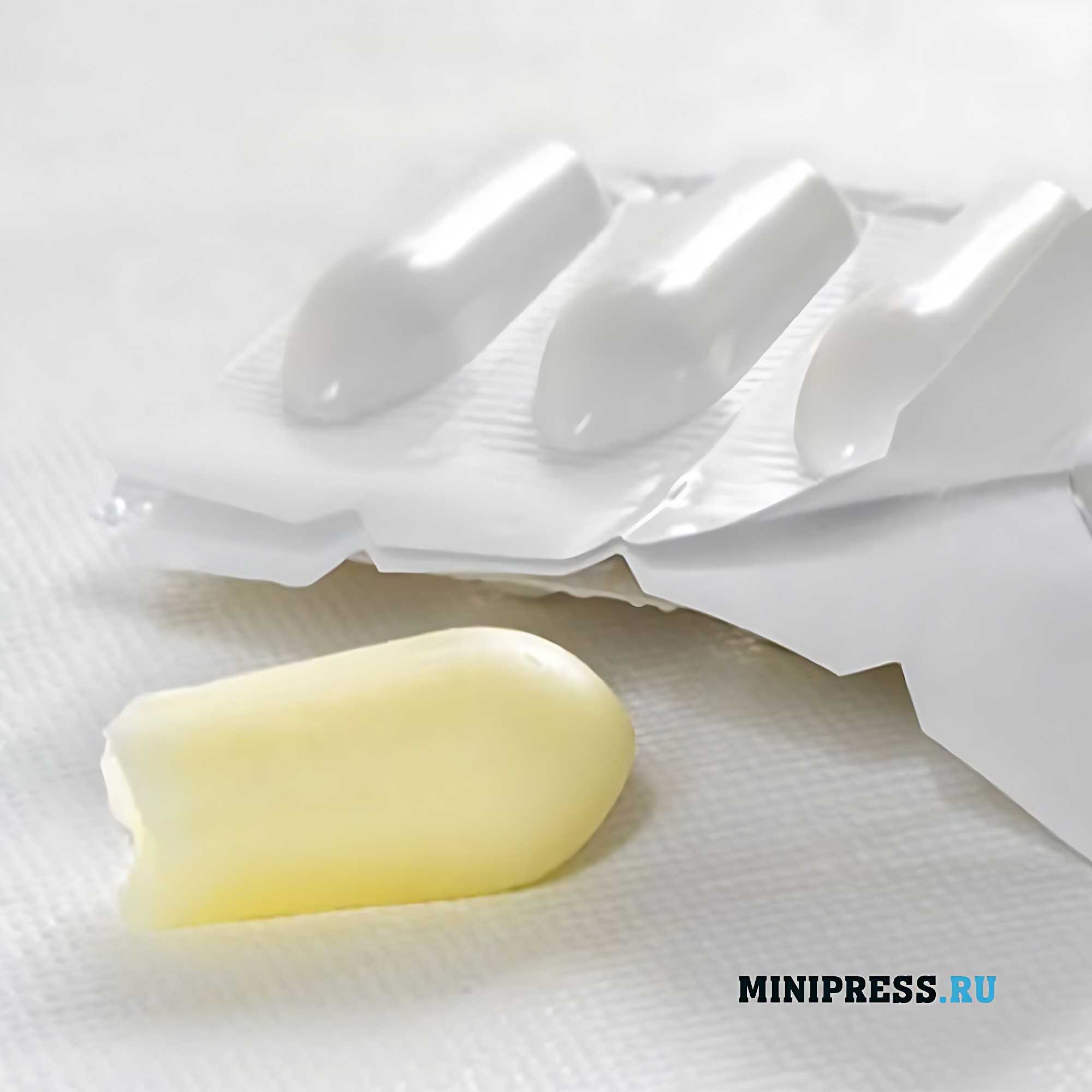
All suppositories (especially rectal and urethral) must have sufficient hardness to overcome the resistance of tissues and sphincters, otherwise the suppositories are deformed and their use becomes impossible. The melting point determined for lipophilic suppositories should not exceed 37 °C. Moreover, it is necessary that their melting occurred in a short temperature range (1-2 ° C). If the definition of melting temperature causes difficulties, then set the time of full deformation, which should be no less than 3 and no more than 15 min. For suppositories made on hydrophilic bases, determine the time of dissolution in water, which should not exceed 1 h.
 Additional information
Additional information
The liquid mass formed after melting or dissolution of suppositories should spontaneously spread over the mucous membrane, forming a uniform layer. This condition is necessary to ensure close contact of the drug substance with tissues and its proper absorption. Suppositories should easily release the drug substances included in them. This quality depends both on the properties of the base used for the preparation of suppositories, and on the method of introduction of drugs into the base. Hydrophilic bases (except polyethylene oxides) easily release active substances, as they are able to dissolve in the secretions of mucous membranes.
Order status tracking
Today we were promised delivery of a blister machine for MN-14 tablets and capsules, remember us ? No one's called us back. 17/07/2025 00:25
Hello Alexander, Our manager Natalya tomorrow until 17:30 will dial the driver, also we ask you yourself to the transportation company. 17/07/2025 00:28
We're from New Jersey, we paid for the equipment on your invoice yesterday. Has the payment been received? How long will it take to deliver to us? 17/07/2025 00:35
James, hello. Payment is credited to our settlement account. Term of manufacture of your equipment by the factory 20 working days, delivery from the factory to our warehouse in the USA 1 month, delivery from the warehouse in Miami 15 calendar days. 17/07/2025 00:36
Granulator for wet granulation ZL-25 and Mixer V-shaped for powders VM-05 under contract No. 15 inform. 17/07/2025 00:45
Hello Emilia, The shipment has arrived at the customs terminal, In the afternoon is scheduled to be transferred to the transportation company. 17/07/2025 00:47
Good day , Electronic conveyor belt package weight detector DK-05 when will we receive in Vatican City ? 17/07/2025 00:55
Hello Grace, your shipment is in the customs warehouse, in the morning your manager Natalia will contact you. 17/07/2025 00:59
Excuse me, I have important information, please do not deliver my shipment, automatic filling and sealing machine for glass ampoules ALG-10 to St. Gallen, but immediately upon arrival send it to Dublin. 17/07/2025 01:05
Hello Emma, we will try to resolve this issue for you. 17/07/2025 01:08
HX-6 Plastic Tube Filling and Sealing Machine in Zagreb? Price delivery terms and conditions 17/07/2025 01:15
Henry, good afternoon, we have received your request by email. In 15 minutes we will send you our quotation for this model of equipment. 17/07/2025 01:17
Have you received payment from our company ? When can we expect delivery ? Please specify realistic terms. 17/07/2025 01:25
Good afternoon Joseph, You paid a few hours ago. We told you that the delivery time is 30 to 45 days. as agreed we will get a reply from the manufacturer on the final time frame. 17/07/2025 01:26
You have to pay for delivery to Bordeaux over and above the delivery amount in the contract ? 17/07/2025 01:35
Elijah, The catalog shows the price with door delivery. The unloading of the equipment, upon receipt of the shipment, will be charged to you separately. 17/07/2025 01:35
Vacuum lyophilic freeze dryer LG-30, can we get faster than the contract ? 17/07/2025 01:45
Hello Penelope, Shipments go through several time stages. Manufacturing, delivery from the factory to the warehouse in Beijing or Shanghai, waiting for batch collection, customs clearance and delivery. It's hard to predict. 17/07/2025 01:46
In Alexandrovka ITA-01 tablet and capsule counter, RZ-9 rotary tablet press and manual capsule filling machine. Help when we get it ? 17/07/2025 01:55
Good afternoon, Mia! We have received from the factories photos of the equipment after packing. Natalia will forward the photos to you. Delivery time to you is 45-50 days. 17/07/2025 01:55
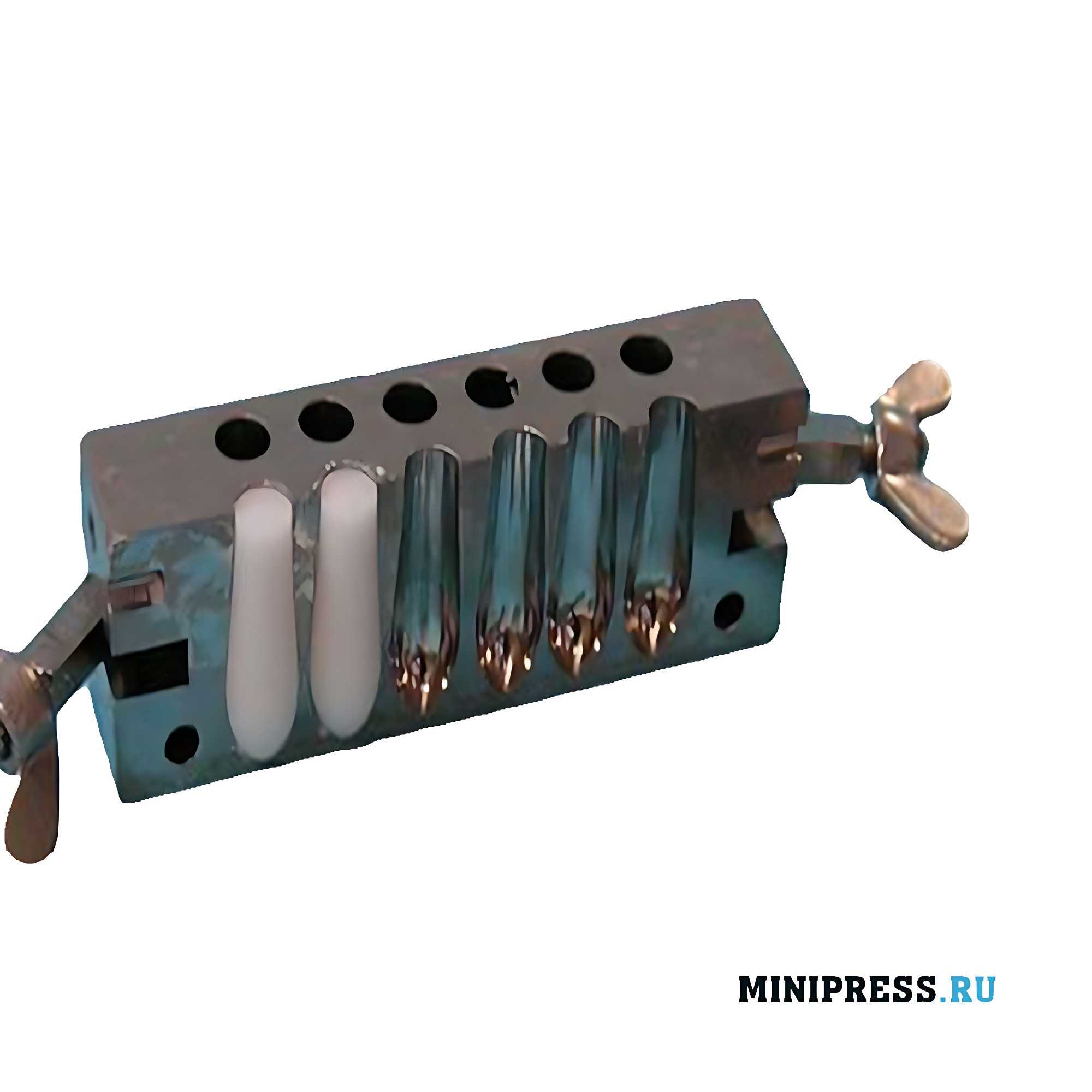
- MACHINES FOR THE PRODUCTION OF SUPPOSITORIES
- HIGH-PRECISION DOSING MACHINES POWDER FILLING MACHINES
- EQUIPMENT FOR PACKING POWDERS INTO VIALS
- EQUIPMENT FOR FILLING CREAMS AND SEALING PLASTIC TUBES
- EQUIPMENT FOR COATING TABLETS
- EQUIPMENT FOR WASHING AND STERILIZING BOTTLES
- EQUIPMENT FOR POLISHING AND DEDUSTING TABLETS AND CAPSULES
- EQUIPMENT FOR FILLING HARD GELATIN CAPSULES WITH POWDER
- BOTTLE FILLING AND CAPPING EQUIPMENT
- AUTOMATIC EQUIPMENT FOR REMOVING TABLETS AND CAPSULES FROM BLISTERS
- EQUIPMENT FOR THE PRODUCTION OF TABLETS
- EQUIPMENT FOR COUNTING AND PACKAGING TABLETS AND CAPSULES IN BOTTLES
- MACHINES FOR FORMING AND FILLING PLASTIC AMPOULES
- EQUIPMENT FOR PACKAGING TABLETS AND CAPSULES IN PLASTIC BOTTLES
- EQUIPMENT FOR PRINTING LOGO ON TABLETS AND CAPSULES
- SPRAY DRYING EQUIPMENT FOR SUSPENSIONS
- EQUIPMENT FOR FILLING AND SEALING GLASS AMPOULES
- EQUIPMENT FOR VACUUM TRANSPORTATION OF POWDERS
- EQUIPMENT FOR SCREW FEEDING OF POWDERS
- EQUIPMENT FOR AUTOMATIC BOTTLE FEEDING FOR FILLING LINES
- AUTOMATIC PHARMACEUTICAL CENTRIFUGES
- EQUIPMENT FOR HOMOGENIZING CREAMS AND OINTMENTS
- EQUIPMENT FOR EFFICIENT MIXING OF POWDERS
- POWDER GRANULATION EQUIPMENT
- EQUIPMENT FOR METAL DETECTOR IN GELATIN CAPSULES AND TABLETS
- FLOW-PACK PACKAGING MACHINES
- AUTOMATIC EQUIPMENT FOR SELF-ADHESIVE LABELS ON PACKAGING
- EQUIPMENT FOR PACKING TEA INTO TEA BAGS WITH THREAD AND LABEL
- EQUIPMENT FOR WEIGHT CONTROL AND SORTING OF CARDBOARD BOXES WITH MEDICINE
- EQUIPMENT FOR VACUUM PACKAGING IN PLASTIC BAGS
- EQUIPMENT FOR THE MANUFACTURE AND PACKAGING OF WET ALCOHOL WIPES
- AUTOMATIC EQUIPMENT FOR BLISTER PACKAGING
- EQUIPMENT FOR FILLING AND PACKAGING HERBAL TINCTURES
- EQUIPMENT FOR PACKAGING FOOD PRODUCTS IN DOY-PACK PACKAGES
- EQUIPMENT FOR PACKAGING BULK MATERIALS IN PLASTIC BAGS
- EQUIPMENT FOR FILLING LIQUIDS IN PLASTIC AND METAL BARRELS
- EQUIPMENT FOR PACKAGING PRODUCTS IN A FLOW PACK
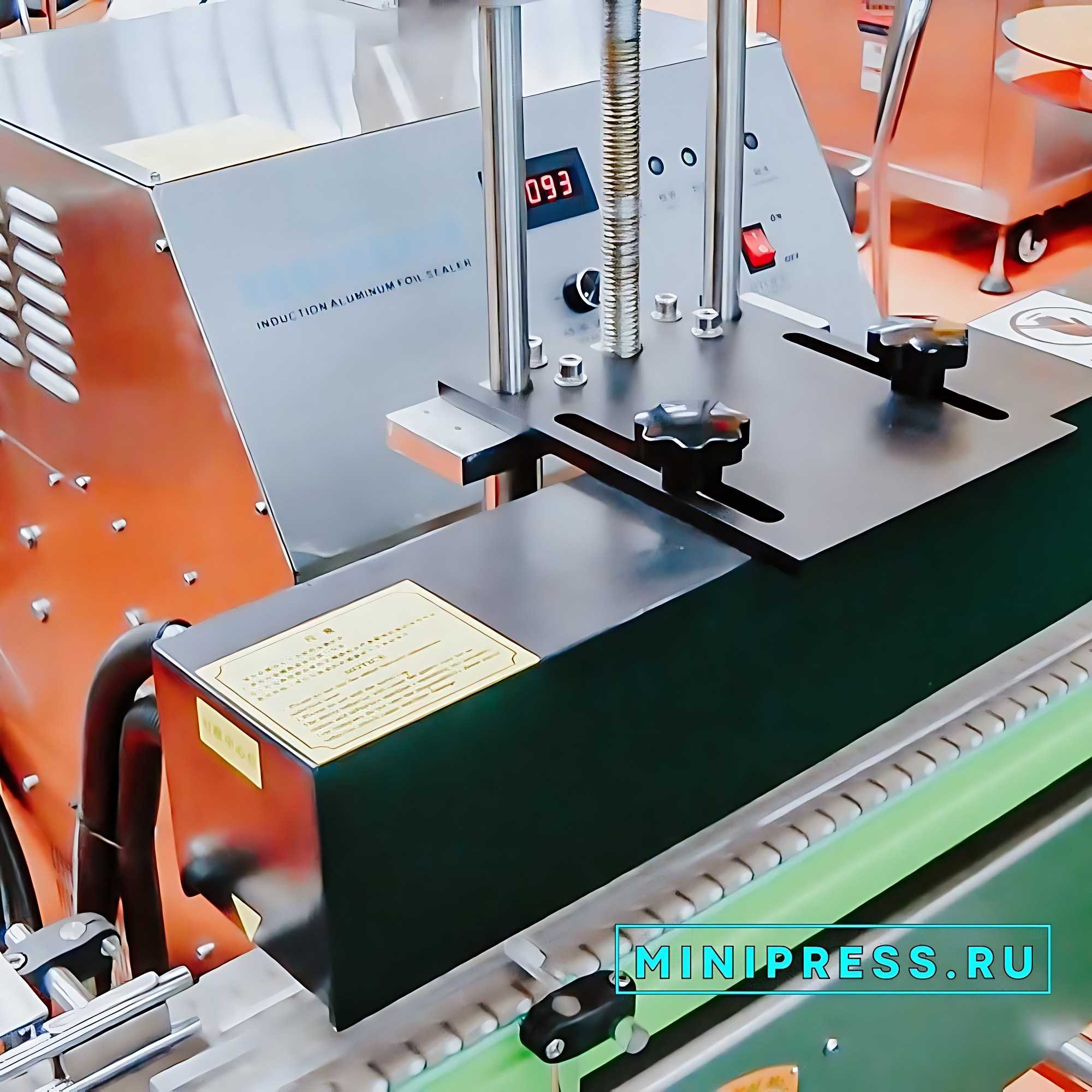
- EQUIPMENT FOR INDUCTION SEALING OF ALU FOIL BOTTLES
- EQUIPMENT FOR WRAPPING CARDBOARD BOXES WITH CELLOPHANE
- EQUIPMENT FOR APPLYING THE EXPIRATION DATE AND BATCH NUMBER TO PRODUCTS
- EQUIPMENT FOR PACKAGING TABLETS IN STRIPS AND TUBES
- DESKTOP EQUIPMENT FOR LIQUID DOSING
- MACHINES PRINTING EXPIRATION DATE AND LOT NUMBER
- EQUIPMENT FOR THE PRODUCTION OF FISHING BOILIES
- DESKTOP EQUIPMENT FOR HIGH-SPEED EMULSION PRODUCTION
- SEMI-AUTOMATIC EQUIPMENT FOR BLISTER PACKAGING
- EQUIPMENT FOR VIBRATING SIEVING OF POWDERS
- MANUAL EQUIPMENT FOR FILLING GELATIN CAPSULES WITH POWDER
- DESKTOP EQUIPMENT FOR MIXING POWDERS
- SEMI-AUTOMATIC EQUIPMENT FOR FILLING GELATIN CAPSULES
- EQUIPMENT FOR MIXING LIQUIDS WITH MICROWAVE HEATING
- EQUIPMENT FOR LABORATORY TESTING OF MEDICINES
- EQUIPMENT FOR AUTOMATIC DOSING OF CREAMS AND OINTMENTS
- PERISTALTIC PUMPS DISPENSERS
- EQUIPMENT FOR POWDERING PHARMA RAW MATERIALS
- EQUIPMENT FOR POLISHING AND DEDUSTING TABLETS AND CAPSULES
- EQUIPMENT FOR PACKAGING TABLETS AND CAPSULES IN PLASTIC BOTTLES
- EQUIPMENT FOR COATING TABLETS
- EQUIPMENT FOR FILLING CREAMS AND SEALING PLASTIC TUBES
- MACHINES FOR THE PRODUCTION OF SUPPOSITORIES
- EQUIPMENT FOR THE PRODUCTION OF TABLETS
- EQUIPMENT FOR FILLING HARD GELATIN CAPSULES WITH POWDER
- EQUIPMENT FOR PACKING POWDERS INTO VIALS
- AUTOMATIC EQUIPMENT FOR REMOVING TABLETS AND CAPSULES FROM BLISTERS
- EQUIPMENT FOR COUNTING AND PACKAGING TABLETS AND CAPSULES IN BOTTLES
- SPRAY DRYING EQUIPMENT FOR SUSPENSIONS
- BOTTLE FILLING AND CAPPING EQUIPMENT
- EQUIPMENT FOR FILLING AND SEALING GLASS AMPOULES
- EQUIPMENT FOR PRINTING LOGO ON TABLETS AND CAPSULES
- EQUIPMENT FOR WASHING AND STERILIZING BOTTLES
- MACHINES FOR FORMING AND FILLING PLASTIC AMPOULES
- HIGH-PRECISION DOSING MACHINES POWDER FILLING MACHINES
- EQUIPMENT FOR AUTOMATIC BOTTLE FEEDING FOR FILLING LINES
- AUTOMATIC PHARMACEUTICAL CENTRIFUGES
- POWDER GRANULATION EQUIPMENT
- EQUIPMENT FOR EFFICIENT MIXING OF POWDERS
- EQUIPMENT FOR HOMOGENIZING CREAMS AND OINTMENTS
- EQUIPMENT FOR VACUUM TRANSPORTATION OF POWDERS
- EQUIPMENT FOR SCREW FEEDING OF POWDERS
- EQUIPMENT FOR WRAPPING CARDBOARD BOXES WITH CELLOPHANE
- EQUIPMENT FOR PACKAGING PRODUCTS IN A FLOW PACK
- EQUIPMENT FOR FILLING AND PACKAGING HERBAL TINCTURES
- EQUIPMENT FOR METAL DETECTOR IN GELATIN CAPSULES AND TABLETS
- EQUIPMENT FOR PACKAGING TABLETS IN STRIPS AND TUBES
- AUTOMATIC EQUIPMENT FOR SELF-ADHESIVE LABELS ON PACKAGING
- EQUIPMENT FOR PACKAGING BULK MATERIALS IN PLASTIC BAGS
- EQUIPMENT FOR PACKING TEA INTO TEA BAGS WITH THREAD AND LABEL
- FLOW-PACK PACKAGING MACHINES
- AUTOMATIC EQUIPMENT FOR BLISTER PACKAGING
- EQUIPMENT FOR FILLING LIQUIDS IN PLASTIC AND METAL BARRELS
- EQUIPMENT FOR VACUUM PACKAGING IN PLASTIC BAGS
- EQUIPMENT FOR THE MANUFACTURE AND PACKAGING OF WET ALCOHOL WIPES
- EQUIPMENT FOR APPLYING THE EXPIRATION DATE AND BATCH NUMBER TO PRODUCTS
- EQUIPMENT FOR WEIGHT CONTROL AND SORTING OF CARDBOARD BOXES WITH MEDICINE
- EQUIPMENT FOR PACKAGING FOOD PRODUCTS IN DOY-PACK PACKAGES
- EQUIPMENT FOR INDUCTION SEALING OF ALU FOIL BOTTLES
- EQUIPMENT FOR MIXING LIQUIDS WITH MICROWAVE HEATING
- MACHINES PRINTING EXPIRATION DATE AND LOT NUMBER
- EQUIPMENT FOR AUTOMATIC DOSING OF CREAMS AND OINTMENTS
- PERISTALTIC PUMPS DISPENSERS
- DESKTOP EQUIPMENT FOR MIXING POWDERS
- MANUAL EQUIPMENT FOR FILLING GELATIN CAPSULES WITH POWDER
- EQUIPMENT FOR VIBRATING SIEVING OF POWDERS
- SEMI-AUTOMATIC EQUIPMENT FOR FILLING GELATIN CAPSULES
- DESKTOP EQUIPMENT FOR HIGH-SPEED EMULSION PRODUCTION
- EQUIPMENT FOR POWDERING PHARMA RAW MATERIALS
- EQUIPMENT FOR THE PRODUCTION OF FISHING BOILIES
- SEMI-AUTOMATIC EQUIPMENT FOR BLISTER PACKAGING
- EQUIPMENT FOR LABORATORY TESTING OF MEDICINES
- DESKTOP EQUIPMENT FOR LIQUID DOSING
 8074
8074 7176739
7176739