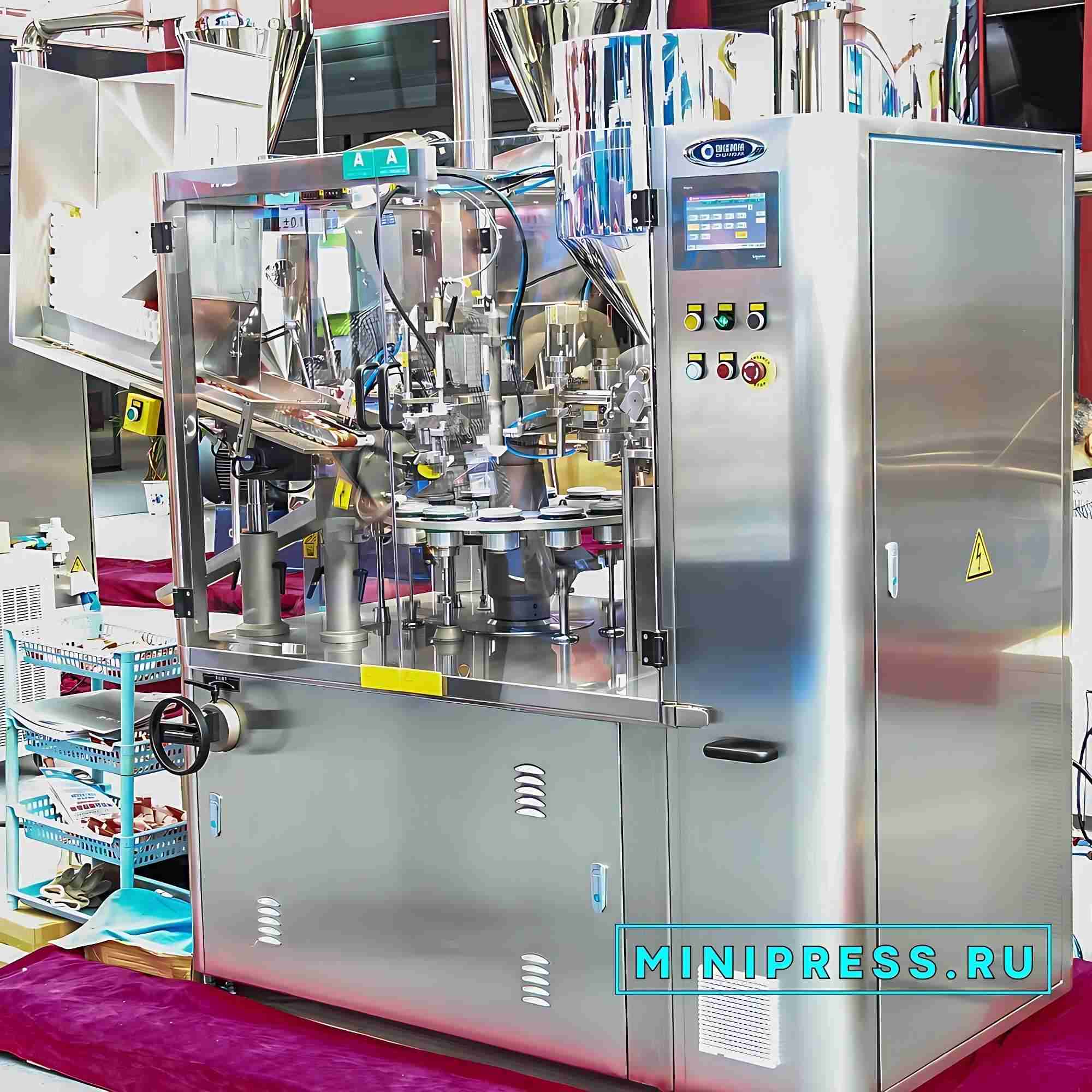
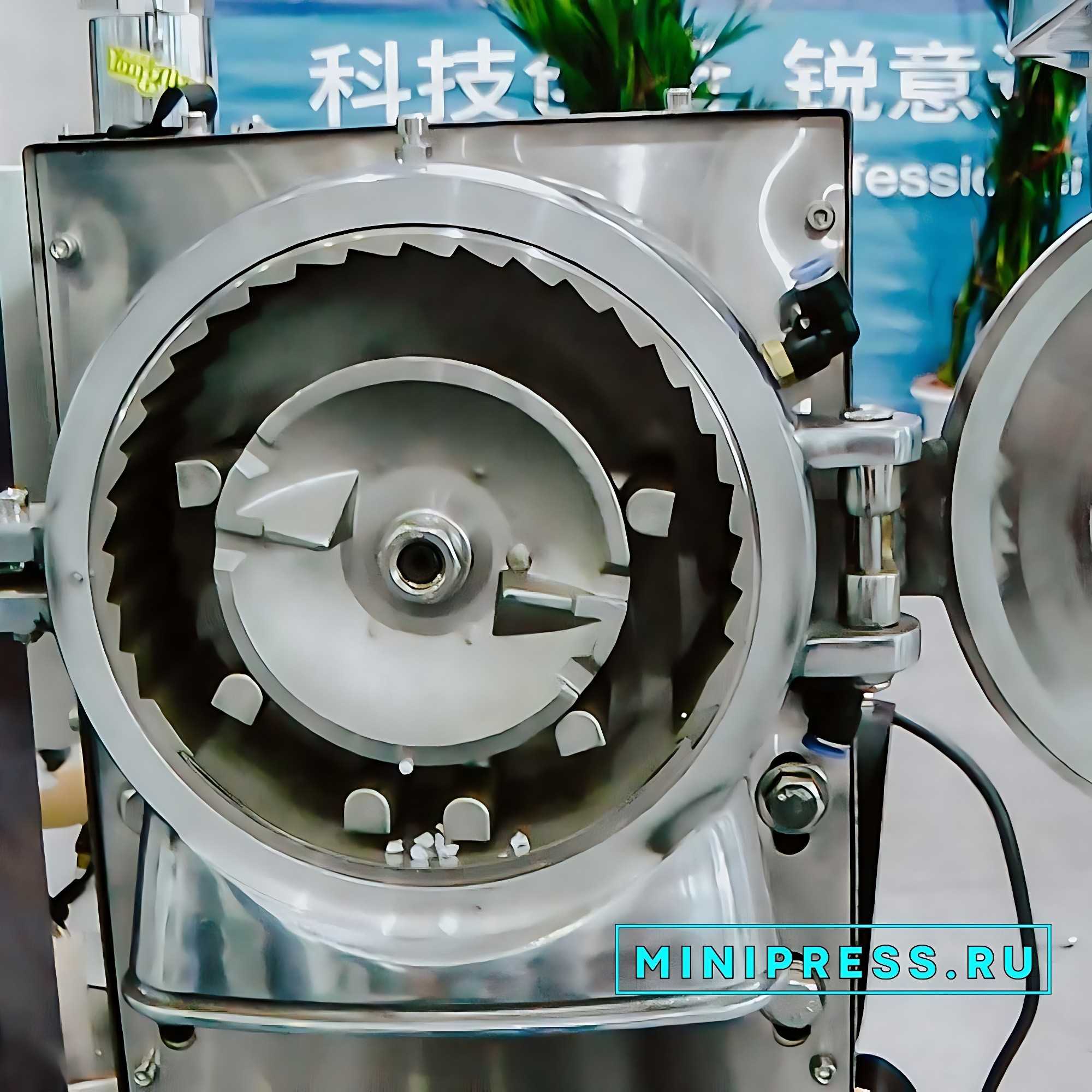
Revisión en video del modelo
Revisión en video del modelo
 Nuestro servicio y atención al cliente
Nuestro servicio y atención al cliente
Abroad, polyethylene glycol bases are known as «Carbovax» (USA), «Scourol» (France), «Postonal», «Suppopharm» (Germany and Scandinavian countries). This group of bases is able to dissolve in the secretions of mucous membranes, fully release medecine substances without irritating the mucosa. These bases have a long shelf life, high physiologic indifference and relatively affordable cost. Gelatin-glycerin and soap-glycerin bases are much less frequently used in the manufacture of suppositories, although they are included in the pharmacopoeias of a number of countries around the world. The following requirements are imposed on hydrophilic suppository bases: to dissolve quickly and completely in the secretions of mucous membranes; not to irritate the mucosa; to mix with hydrophobic medecine substances or absorb them; to be chemically and pharmacologically indifferent.
For suppositories made on hydrophilic bases, determine the dissolution time. To do this, one suppository is placed at the bottom of a vessel with a capacity of 100 ml, containing 50 ml of water with a temperature of (37±1) °C. Every 5 min the vessel is shaken so that the liquid and the sample acquire rotational motion. The suppository should dissolve within 1h. To ensure optimal structural and mechanical characteristics of suppository bases to them add stearates of aluminum, magnesium and other salts of fatty acids, as well as emulsifiers T-1, T-2 and №1, bentonite, glucose, starch, aerosil and other auxiliary substances approved for medical use.
 Glosario Farmacéutico
Glosario Farmacéutico
 Especificaciones técnicas
Especificaciones técnicas
Closely related to these general requirements and technological requirements for bases, which should: show chemical and physical stability in the process of manufacture and storage of suppositories, easily molded and retain the necessary hardness during insertion, have the ability to emulsify the required amount of solutions, have a certain plasticity, viscosity, deformation time and structural and mechanical properties. These technological requirements are met by lipophilic, hydrophilic bases and their mixtures used in the pharmaceutical industry in different countries of the world. As lipophilic suppository bases State Pharmacopoeia USSR XI recommends using cocoa butter, its alloys with paraffin and hydro-genated fats, vegetable and animal fats, solid fat, lanol, alloys of fats with wax and solid paraffin. Currently, cocoa butter remains the official pharmacopoeial base in the pharmacopoeias of a number of countries. Lipophilic bases should meet the following requirements: quickly melt in the rectum, have a melting point not higher than 37 ° C, have sufficient hardness and a small difference between the melting and solidification temperatures, have sufficient viscosity, absorb liquids well, be stable during storage.
 Información adicional
Información adicional
Due to the fact that the melting points of the base components fluctuate within a fairly wide range (hydrofat – from 28 to 37 ° C, paraffin – from 50 to 57 ° C, cocoa butter – from 30 to 34 ° C), the actual melting point of the resulting alloy may be slightly higher or lower than the specified temperature. In this case, paraffin or hydrofat is added to the base in the amounts necessary to bring the melting temperature of the mixture to the specified value. In this case, use the «table of additives». In addition to the required melting point, suppository base should also have appropriate structural and mechanical properties. They can be judged by the time of total deformation of the suppository prepared from this base. Determination of the time of full deformation is carried out at a temperature of 37 ° C on the device Kruvchinsky. In the composition of bases are often introduced surfactants, which not only improve the structural and mechanical properties, but also affect the kinetics of release and absorption of medecine. Hydrophilic suppository bases are mainly represented by polyethylene glycols (polyethylene oxides) – condensed polymers of ethylene oxide and water. Domestic industry produces polyethylene glycols differing in molecular weight: PEO-400, PEO-1500, PEO-2000, PEO-4000 and PEO-6000.
Seguimiento del estado del pedido
Horizontal granulator for organic fertilizers JU-80, the price is up to date ? Delivery time to York? Urgent. 13/05/2024 00:27
Ethan, Please read carefully the description of this model in our catalog. Prices are current. Timing 1-1.5 months. 13/05/2024 00:30
Horizontal granulator for organic fertilizers JU-80, the price is up to date ? Delivery time to Istanbul? Urgent. 13/05/2024 00:37
Joseph, Please read carefully the description of this model in our catalog. Prices are current. Timing 1-1.5 months. 13/05/2024 00:41
How to contact you for shipment tracking ? Industrial vibrating screen VS-04. 13/05/2024 00:47
Good afternoon, Lily, My WhastApp is +79853643808, Manager Natalia +79153808881 during business hours and except weekends. 13/05/2024 00:50
Today we were promised delivery of a blister machine for MN-14 tablets and capsules, remember us ? No one's called us back. 13/05/2024 00:57
Hello Savannah, Our manager Natalya tomorrow until 14:30 will dial the driver, also we ask you yourself to the transportation company. 13/05/2024 00:58
Good day, I want tomorrow until 12:30 to get FF-02 tablet and capsule filling machine in Prague? 13/05/2024 01:07
Good afternoon, Ella! We have contacted the transportation company, your shipment is arriving in Prague without delay. Please wait for a call from a representative of Business Lines. 13/05/2024 01:07
Good afternoon, we received the PR-15 powder dispenser in plastic vials, scheduled tomorrow until 16:30 to open the box and test. 13/05/2024 01:17
Hazel, Good afternoon. Great news, please WhatsApp +79853643808 to send photo and video report. 13/05/2024 01:18
Mezclador con paleta para polvos CX-100 ¿cuál es el tamaño exacto del empaque ? 13/05/2024 01:27
Riley, ¡Hola! Dimensiones del embalaje 1200 x 640 x 1000 mm, peso del embalaje 250 kg. Por favor, avíseme cuando reciba el envío. 13/05/2024 01:30
El gerente me dice que la máquina etiquetadora automática de doble cara ST-36 ha salido de fábrica, factura # 118. 13/05/2024 01:37
Hola Amelia, El equipo se envió desde el almacén de tránsito y la entrega se está realizando según lo planeado. No se esperan retrasos. 13/05/2024 01:37
Buenos días, tiempo de entrega de la máquina llenadora de tambor semiautomática DF - 10 a Ciudad del Carmen, ¿nos llamará el servicio de mensajería? 13/05/2024 01:47
Avery, buenas tardes. Natalia se pondrá en contacto contigo, el envío ha pasado el despacho de aduana en la frontera. 13/05/2024 01:47
Granulador de polvo seco y húmedo YR-90 El mes YR-90 ya pasó. Apresúrate a la fábrica. 13/05/2024 01:57
Emilia, buenas tardes. La fábrica ha enviado su equipo a nuestro representante, ahora está formando un envío consolidado para su envío a la aduana. Te mantendremos informado. 13/05/2024 02:00
- EQUIPO PARA LLENADO Y SELLADO DE AMPOLLAS DE VIDRIO
- EQUIPO PARA LAVAR Y ESTERILIZAR BIBERONES
- EQUIPO PARA LLENADO DE CREMAS Y SELLADO DE TUBOS DE PLÁSTICO
- EQUIPO PARA EL ENVASADO DE COMPRIMIDOS Y CÁPSULAS EN BOTELLAS DE PLÁSTICO
- EQUIPO DE LLENADO Y TAPADO DE BOTELLAS
- EQUIPO PARA LLENAR CÁPSULAS DE GELATINA DURA CON POLVO
- MÁQUINAS PARA LA PRODUCCIÓN DE SUPOSITORIOS
- MÁQUINAS PARA FORMAR Y LLENAR AMPOLLAS DE PLÁSTICO
- EQUIPO DE SECADO POR PULVERIZACIÓN PARA SUSPENSIONES
- MÁQUINAS DOSIFICADORAS DE ALTA PRECISIÓN MÁQUINAS DE LLENADO DE POLVO
- EQUIPO PARA ENVASAR POLVOS EN VIALES
- EQUIPO PARA IMPRIMIR LOGOTIPOS EN TABLETAS Y CÁPSULAS.
- EQUIPO PARA CONTAR Y ENVASAR COMPRIMIDOS Y CÁPSULAS EN FRASCOS
- EQUIPO PARA LA PRODUCCIÓN DE TABLETAS
- EQUIPO AUTOMÁTICO PARA EXTRAER COMPRIMIDOS Y CÁPSULAS DE BLÍSTERES.
- EQUIPO PARA PULIR Y DESEMPOLVAR TABLETAS Y CÁPSULAS
- EQUIPO PARA RECUBRIMIENTO DE TABLETAS
- EQUIPO PARA ALIMENTACIÓN AUTOMÁTICA CON BIBERÓN PARA LÍNEAS DE LLENADO
- EQUIPO PARA TRANSPORTE DE POLVOS AL VACÍO
- EQUIPO DE GRANULACIÓN DE POLVO
- CENTRIFUGADORAS FARMACÉUTICAS AUTOMÁTICAS
- EQUIPO PARA ALIMENTACIÓN POR TORNILLO DE POLVOS
- EQUIPO PARA HOMOGENEIZAR CREMAS Y UNGÜENTOS
- EQUIPO PARA LA MEZCLA EFICIENTE DE POLVOS
- EQUIPO PARA EMPACAR TÉ EN BOLSITAS DE TÉ CON HILO Y ETIQUETA.
- EQUIPO AUTOMÁTICO PARA ETIQUETAS AUTOADHESIVAS EN ENVASES
- EQUIPO PARA ENVOLVER CAJAS DE CARTÓN CON CELOFÁN
- EQUIPO PARA ENVASAR PRODUCTOS EN UN FLOW PACK
- EQUIPO AUTOMÁTICO PARA ENVASADO EN BLÍSTER
- EQUIPOS PARA EL ENVASADO DE COMPRIMIDOS EN TIRAS Y TUBOS
- EQUIPOS PARA ENVASADO AL VACÍO EN BOLSAS DE PLÁSTICO
- EQUIPO PARA LA FABRICACIÓN Y ENVASADO DE TOALLITAS HÚMEDAS CON ALCOHOL
- EQUIPO PARA APLICAR LA FECHA DE CADUCIDAD Y EL NÚMERO DE LOTE A LOS PRODUCTOS.
- EQUIPO PARA SELLADO POR INDUCCIÓN DE BOTELLAS DE ALUMINIO
- MÁQUINAS DE ENVASADO FLOW-PACK
- EQUIPOS PARA EL ENVASADO DE MATERIALES A GRANEL EN BOLSAS DE PLÁSTICO
- EQUIPO PARA CONTROL DE PESO Y CLASIFICACIÓN DE CAJAS DE CARTÓN CON MEDICAMENTOS
- EQUIPOS PARA EL ENVASADO DE PRODUCTOS ALIMENTICIOS EN ENVASES DOY-PACK
- EQUIPO PARA EL LLENADO Y ENVASADO DE TINTURAS DE HIERBAS
- EQUIPO PARA DETECTOR DE METALES EN CÁPSULAS Y TABLETAS DE GELATINA
- EQUIPOS PARA LLENADO DE LÍQUIDOS EN BARRILES DE PLÁSTICO Y METAL.
- EQUIPO PARA LA PRODUCCIÓN DE BOILIES DE PESCA
- EQUIPO PARA DOSIFICACIÓN AUTOMÁTICA DE CREMAS Y UNGÜENTOS
- EQUIPO DE SOBREMESA PARA LA PRODUCCIÓN DE EMULSIONES A ALTA VELOCIDAD
- DISPENSADORES DE BOMBAS PERISTÁLTICAS
- EQUIPO PARA PRUEBAS DE LABORATORIO DE MEDICAMENTOS
- EQUIPOS DE SOBREMESA PARA DOSIFICACIÓN DE LÍQUIDOS
- EQUIPO PARA PULVERIZAR MATERIAS PRIMAS FARMACÉUTICAS
- EQUIPO SEMIAUTOMÁTICO PARA ENVASADO DE BLÍSTERES
- EQUIPO PARA TAMIZADO VIBRATORIO DE POLVOS
- MÁQUINAS QUE IMPRIMEN FECHA DE CADUCIDAD Y NÚMERO DE LOTE
- EQUIPO PARA MEZCLAR LÍQUIDOS CON CALENTAMIENTO POR MICROONDAS
- EQUIPO MANUAL PARA LLENAR CÁPSULAS DE GELATINA CON POLVO.
- EQUIPO DE SOBREMESA PARA MEZCLAR POLVOS
- EQUIPO SEMIAUTOMÁTICO PARA EL LLENADO DE CÁPSULAS DE GELATINA
- EQUIPO AUTOMÁTICO PARA EXTRAER COMPRIMIDOS Y CÁPSULAS DE BLÍSTERES.
- MÁQUINAS DOSIFICADORAS DE ALTA PRECISIÓN MÁQUINAS DE LLENADO DE POLVO
- MÁQUINAS PARA FORMAR Y LLENAR AMPOLLAS DE PLÁSTICO
- EQUIPO PARA LLENADO Y SELLADO DE AMPOLLAS DE VIDRIO
- EQUIPO DE LLENADO Y TAPADO DE BOTELLAS
- EQUIPO PARA IMPRIMIR LOGOTIPOS EN TABLETAS Y CÁPSULAS.
- EQUIPO PARA LLENAR CÁPSULAS DE GELATINA DURA CON POLVO
- MÁQUINAS PARA LA PRODUCCIÓN DE SUPOSITORIOS
- EQUIPO PARA LLENADO DE CREMAS Y SELLADO DE TUBOS DE PLÁSTICO
- EQUIPO PARA ENVASAR POLVOS EN VIALES
- EQUIPO PARA RECUBRIMIENTO DE TABLETAS
- EQUIPO PARA PULIR Y DESEMPOLVAR TABLETAS Y CÁPSULAS
- EQUIPO PARA LAVAR Y ESTERILIZAR BIBERONES
- EQUIPO DE SECADO POR PULVERIZACIÓN PARA SUSPENSIONES
- EQUIPO PARA EL ENVASADO DE COMPRIMIDOS Y CÁPSULAS EN BOTELLAS DE PLÁSTICO
- EQUIPO PARA CONTAR Y ENVASAR COMPRIMIDOS Y CÁPSULAS EN FRASCOS
- EQUIPO PARA LA PRODUCCIÓN DE TABLETAS
- EQUIPO DE GRANULACIÓN DE POLVO
- EQUIPO PARA LA MEZCLA EFICIENTE DE POLVOS
- EQUIPO PARA ALIMENTACIÓN AUTOMÁTICA CON BIBERÓN PARA LÍNEAS DE LLENADO
- CENTRIFUGADORAS FARMACÉUTICAS AUTOMÁTICAS
- EQUIPO PARA HOMOGENEIZAR CREMAS Y UNGÜENTOS
- EQUIPO PARA ALIMENTACIÓN POR TORNILLO DE POLVOS
- EQUIPO PARA TRANSPORTE DE POLVOS AL VACÍO
- EQUIPO PARA LA FABRICACIÓN Y ENVASADO DE TOALLITAS HÚMEDAS CON ALCOHOL
- EQUIPO PARA DETECTOR DE METALES EN CÁPSULAS Y TABLETAS DE GELATINA
- EQUIPOS PARA ENVASADO AL VACÍO EN BOLSAS DE PLÁSTICO
- EQUIPO PARA EL LLENADO Y ENVASADO DE TINTURAS DE HIERBAS
- EQUIPOS PARA EL ENVASADO DE PRODUCTOS ALIMENTICIOS EN ENVASES DOY-PACK
- EQUIPO PARA ENVASAR PRODUCTOS EN UN FLOW PACK
- MÁQUINAS DE ENVASADO FLOW-PACK
- EQUIPO PARA CONTROL DE PESO Y CLASIFICACIÓN DE CAJAS DE CARTÓN CON MEDICAMENTOS
- EQUIPO PARA ENVOLVER CAJAS DE CARTÓN CON CELOFÁN
- EQUIPOS PARA EL ENVASADO DE MATERIALES A GRANEL EN BOLSAS DE PLÁSTICO
- EQUIPO AUTOMÁTICO PARA ETIQUETAS AUTOADHESIVAS EN ENVASES
- EQUIPOS PARA EL ENVASADO DE COMPRIMIDOS EN TIRAS Y TUBOS
- EQUIPOS PARA LLENADO DE LÍQUIDOS EN BARRILES DE PLÁSTICO Y METAL.
- EQUIPO AUTOMÁTICO PARA ENVASADO EN BLÍSTER
- EQUIPO PARA APLICAR LA FECHA DE CADUCIDAD Y EL NÚMERO DE LOTE A LOS PRODUCTOS.
- EQUIPO PARA EMPACAR TÉ EN BOLSITAS DE TÉ CON HILO Y ETIQUETA.
- EQUIPO PARA SELLADO POR INDUCCIÓN DE BOTELLAS DE ALUMINIO
- EQUIPO SEMIAUTOMÁTICO PARA ENVASADO DE BLÍSTERES
- MÁQUINAS QUE IMPRIMEN FECHA DE CADUCIDAD Y NÚMERO DE LOTE
- EQUIPO PARA MEZCLAR LÍQUIDOS CON CALENTAMIENTO POR MICROONDAS
- EQUIPO PARA DOSIFICACIÓN AUTOMÁTICA DE CREMAS Y UNGÜENTOS
- DISPENSADORES DE BOMBAS PERISTÁLTICAS
- EQUIPO PARA TAMIZADO VIBRATORIO DE POLVOS
- EQUIPO MANUAL PARA LLENAR CÁPSULAS DE GELATINA CON POLVO.
- EQUIPO DE SOBREMESA PARA LA PRODUCCIÓN DE EMULSIONES A ALTA VELOCIDAD
- EQUIPOS DE SOBREMESA PARA DOSIFICACIÓN DE LÍQUIDOS
- EQUIPO PARA LA PRODUCCIÓN DE BOILIES DE PESCA
- EQUIPO PARA PULVERIZAR MATERIAS PRIMAS FARMACÉUTICAS
- EQUIPO PARA PRUEBAS DE LABORATORIO DE MEDICAMENTOS
- EQUIPO DE SOBREMESA PARA MEZCLAR POLVOS
- EQUIPO SEMIAUTOMÁTICO PARA EL LLENADO DE CÁPSULAS DE GELATINA
 7703
7703 5938345
5938345